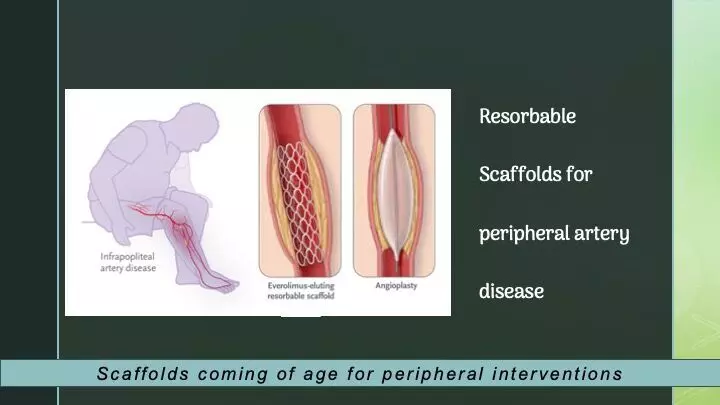
In patients experiencing chronic limb-threatening ischemia (CLTI) coupled with infrapopliteal artery disease, the utilization of angioplasty has been linked to frequent reinterventions and unfavorable limb outcomes due to restenosis. Presenting findings from the LIFE-BTK trial, researchers have demonstrated that employing an everolimus-eluting resorbable scaffold proves superior to angioplasty in achieving positive clinical outcomes, such as freedom from amputation or reintervention within a year. The results were recently published in NEJM.
Endovascular therapy applied to arterial vessels below the knees is known to have inherent limitations, including issues such as elastic recoil, dissection, and restenosis, which compromise the long-term success of the procedure. Atherosclerotic plaque below the knees typically contains higher levels of calcium and luminal thrombus compared to lesions above the knees, potentially influencing the response to therapies more commonly applied in treating upper limb arteries.
Varcoe et al conducted a rigorous single-blind, randomized, controlled trial (LIFE-BTK) to assess the safety and efficacy of a novel everolimus-eluting resorbable scaffold (Esprit BTK, Abbott Vascular) for treating infrapopliteal artery disease in CLTI patients.
During the trial, individuals with CLTI and infrapopliteal artery disease were randomly assigned in a 2:1 ratio to receive either the everolimus-eluting resorbable scaffold or balloon angioplasty. A pivotal eligibility criterion was Rutherford–Becker class 4 or 5 disease, excluding class 6. Additionally, arterial stenosis severity had to be at least 70% of the vessel diameter upon visual assessment.
The primary efficacy endpoint was freedom from specific events at the one-year mark, including amputation above the ankle, complete occlusion of the target vessel, clinically driven revascularization of the target lesion, and binary restenosis of the target lesion. Meanwhile, the primary safety endpoint was freedom from major adverse limb events at six months and perioperative death.
Following a median follow-up period of 390 days, the primary efficacy endpoint (i.e., no events occurred) was observed in 74% of the scaffold group and 44% of the angioplasty group. Subgroup analyses and assessments of other arterial patency endpoints consistently favored the scaffold treatment, serving as promising indicators of success for the evaluated therapy.
“Focus on clinical endpoints”
In an accompanying editorial, Joshua A. Beckman, M.D. has stressed that “…what truly marks this trial as important is not only the arterial patency data but also the clinical end points. The inclusion of clinical end points is less common in studies of new devices for the treatment of vascular disease of the leg than in studies of medications.”
The recently published literature supporting interventional management of peripheral artery disease includes PROMISE II and EMINENET trials. As the field moves forward with new treatments, the incorporation of clinical outcomes should be standard. Only then will clinicians be able to understand the true value of new tools and treatments.
Source: NEJM:
1. DOI: 10.1056/NEJMoa2305637
2. DOI: 10.1056/NEJMe2312167
